The Challenges
Despite tremendous success in treatment of liquid cancers, cell therapies have struggled to provide durable responses in solid cancers, and most patients with advanced solid cancers showing initial response to treatment will eventually relapse. Current cell therapies are made from the patient’s own T cells which is costly, time consuming and inherently difficult to scale. Furthermore, due to the safety profile of these therapies, patients must be admitted to specialist hospitals which further drives costs and limits access to patients.
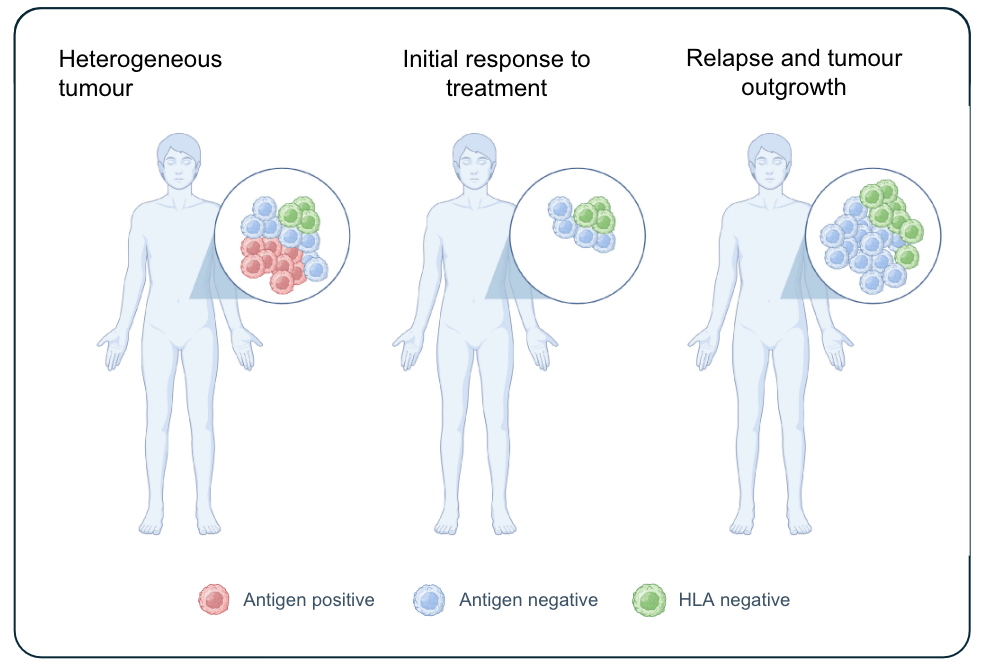
Tumor Heterogeneity
CAR-T or TCR-T cells specifically target cancer cells that express a single protein (e.g. CD19 and MAGE-A4). Cancer cells not expressing this proteins will not be eliminated.
The tumour may escape the therapy and grow back
Scalability
CAR- or TCR-T cells are custom-made. The patient’s own cells are engineered and given back. This is costly, prone to failure and takes precious time from patients
Safety Profile
Due to the toxicity of current cell therapies, patients are treated in specialist centers and not community hospitals limiting global access to these therapies
Tumours are diverse (heterogeneous), evolve over time, and contain a mix of cells with different expression profiles of proteins. Therapies targeting a single protein (CAR-T and TCR-T) can only eliminate the cancer cells expressing that protein. This may lead to an initial response, but cancer cells not expressing the targeted protein will grow back and the patients relapse. This significantly limits the curative potential of current cell therapies.
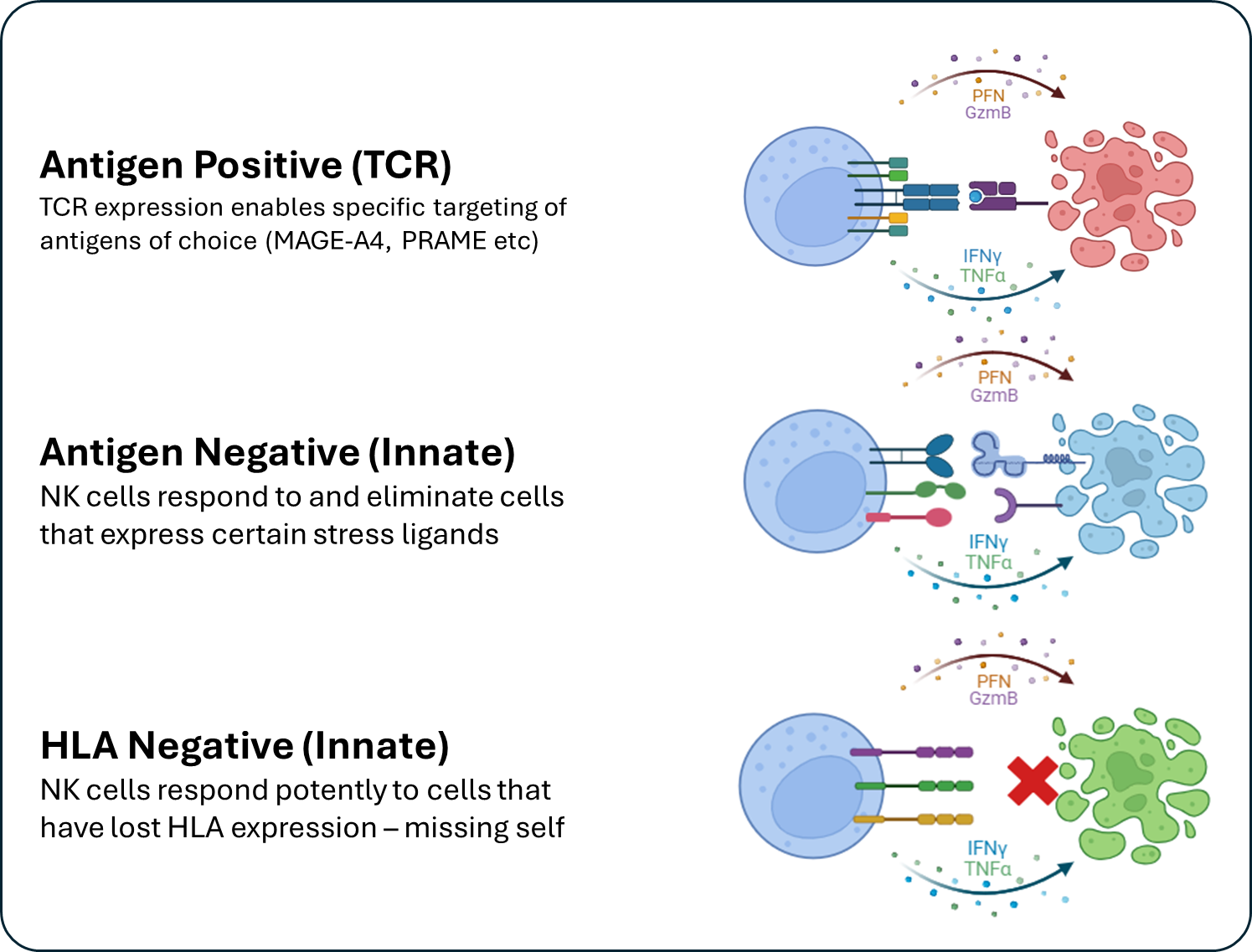
The Solution – TCR-NK
Zelluna’s TCR-NK cells combine the potency, safety profile and scalability of allogeneic “off-the-shelf” NK cells with the exquisite cancer specific targeting of the T cell receptor (TCR). TCR-NK cells can therefore eliminate cancer cells based on the specificity of the TCR and through a multitude of activating receptors that broadly detects cancers. TCR-NK cells can be manufactured upfront at a large scale and shipped to patients on demand, enabling scalability to a large patient population. NK cell therapies also have a favorable safety profile that may enable treatment in an out-patient setting to minimize burden on patients and healthcare systems.
Tumor Heterogeneity
TCR-NK cells can eliminate cancer cells that express the targeted protein and cells that do not express the targeted protein.
Consequently, TCR-NK cells may eradicate heterogeneous tumors
Scalability
TCR-NK cells can be manufactured upfront at large scale using NK cells derived from healthy donors and shipped to patients on demand.
This enables potential treatment of patients on a large scale
Safety Profile
NK cell therapies are considered safe and does not cause toxicities normally seen with CAR-T or TCR-T therapies.
This may enable treatment in all types of hospital settings.
TCR-NK cells combine TCR mediated activity with innate NK activity and can target cancer cells through both the TCR and also through recognition of stress ligands expressed by cancer cells as well as cancer cells that have lost expression of HLA. This means that TCR-NK cells can overcome the escape mechanisms used by cancer cells to escape detection by T cells (antigen loss, HLA loss) and potentially provide long lasting responses in solid cancers